
Doctoral Program in Molecular and Cell Biology
Advanced Courses and Seminars I
COURSE: Cell Biology / Biologia Celular
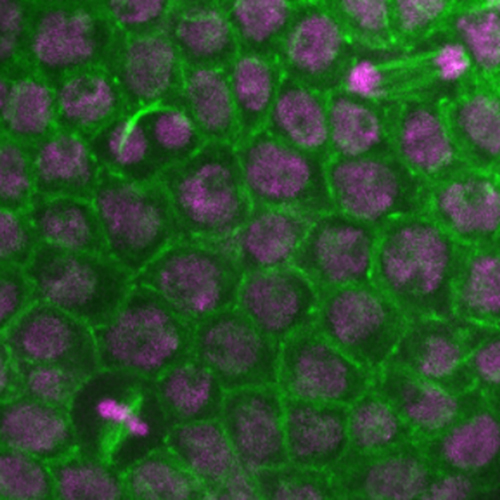
Coordinator(s): Claudio Sunkel
Faculty and invited speakers: Claudio Sunkel, Carlos Conde, Elsa Logarinho and Eurico Morais-de-Sá
Short summary: The cell is the basic unit of life and so understanding cell biology is essential to learn how organisms develop, grow and maintain homeostasis. The cell biology course is focused in cell-cycle control, regulation of chromosome segregation, generation of cell polarity, and also covers how aberrant cellular function contributes for aging. Lectures provide basic knowledge and emerging research in the field.
Outline of the course: 4 lectures
.......................................................................................................
COURSE: Ethics, Science and Society / Ética, Ciência e Sociedade

Coordinator(s): Susana Magalhães
Faculty and invited speakers: Anna Olsson, Júlio Borlido Santos, Susana Magalhães
Short summary: The Ethics, Science and Society module gives students an introduction to ethics and research integrity as well as science with and for society. Focus is on issues of general relevance (e g research integrity, authorship issues, public dialogue) and on an interactive way of teaching.
Outline of the course: 3 lectures
.......................................................................................................
COURSE: Functional Genetics / Genética Funcional
Coordinator(s): Alexandra Moreira and Didier Cabanes
Faculty and invited speakers: Maria João Prata, Carolina Lemos, Isabel Silveira, Alexandra Moreira, Jorge Vieira, Cristina Vieira, Daniel Osório, Sandra Sousa, Didier Cabanes
Short summary: Functional genetics is devoted at unravelling the function of DNA at the level of genes, RNA transcripts and protein products so as to understand the relationship between genome and functional properties, or phenotype, of a given organism. A major focus of functional genetics is the development of treatments for many diseases, namely human genetic diseases. Experts in the area will present state-of-the-art research on functional genetics during the physiological events that occur in different types of cells and in different systems. Scientific challenges in the field and how they are experimentally addressed will be discussed with the students.
Outline of the course: Lectures, journal clubs, research seminars
.......................................................................................................
COURSE: Infection and Immunity / Infeção e Imunidade

Coordinator(s): Nuno Alves, Margarida Saraiva and Pedro Moura Alves
Faculty and invited speakers: Nuno Alves, Alexandre Carmo, Nuno Santarém, Margarida Saraiva, Joana Tavares, Manuel Vilanova, Pedro Moura Alves
Short summary: This course covers the principles underlying the function of the immune system in health and disease. In particular, it will detail the molecular basis of immunity and tolerance induction, and discuss how the understanding of pathogen-host interactions can be therapeutically explored to improve the immune response during infection.
Outline of the course: Lectures
.......................................................................................................
COURSE: Molecular Mechanisms / Mecanismos Moleculares
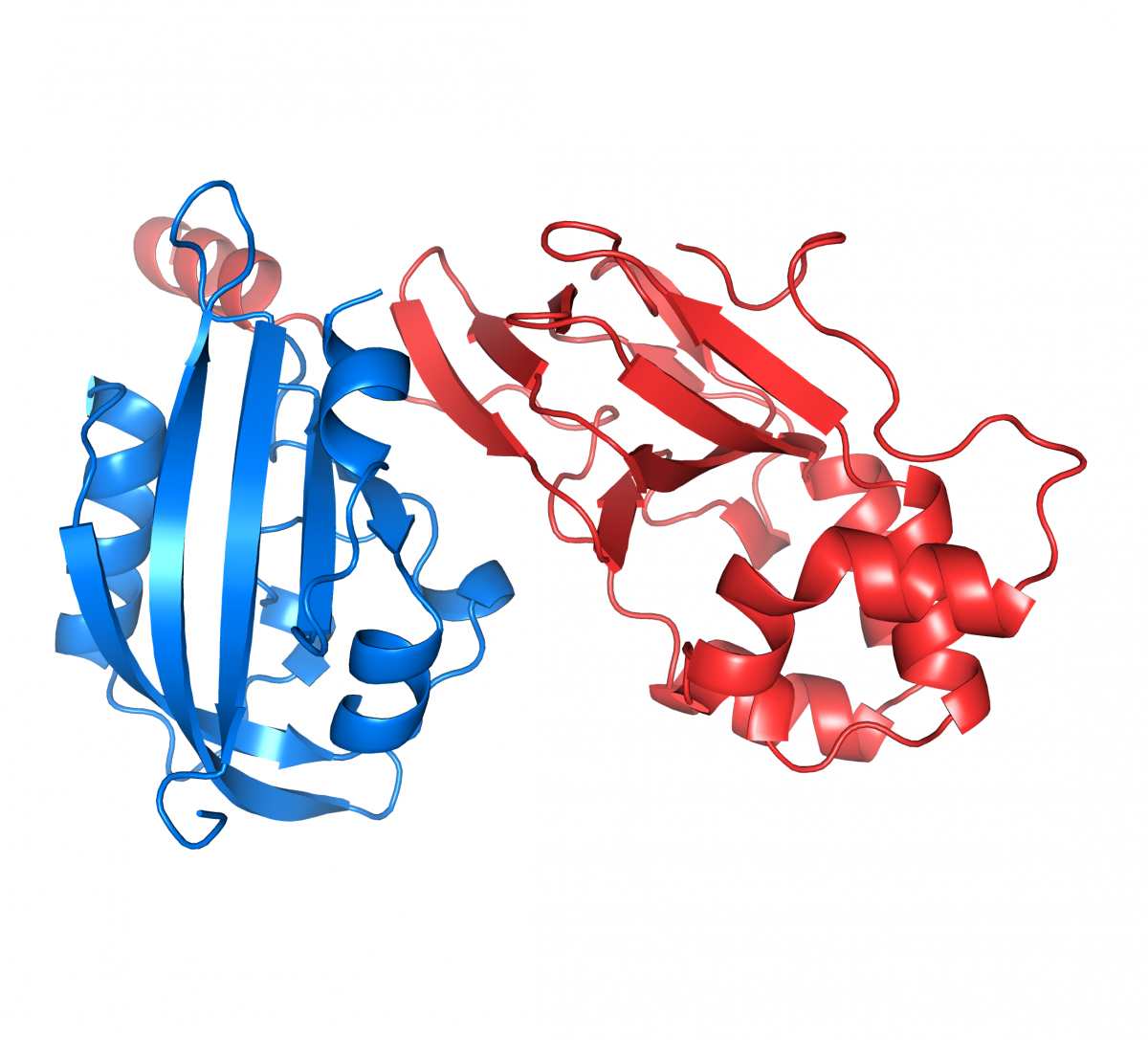
Coordinator(s): João Morais Cabral and Sandra Macedo-Ribeiro
Faculty and invited speakers:
Short summary: This course aims at providing an understanding about molecular mechanism through the analysis of well characterized model systems and exploration of concepts underlying molecular recognition and selectivity, strength of interaction, molecular reaction and molecular regulation.
Outline of the course: Lectures and practical work
.......................................................................................................
COURSE: Neurobiology / Neurobiologia
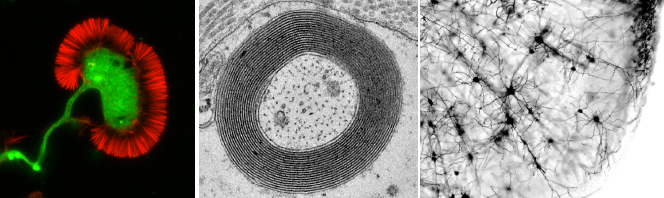
Coordinator(s): Mónica Sousa
Faculty and invited speakers: Mónica Sousa, João Relvas, Pedro Brites, Teresa Summavielle, Boris Safronov, Paulo Aguiar, Matthew Holt, Diogo Castro
Short summary: This course will address different aspects of neuronal and glial cell biology, from axon growth to myelination, synapse formation, cytoskeleton organization and dynamics, mechanisms of addiction and organization of neuronal networks.
Outline of the course: Lectures, group discussions, journal clubs
.......................................................................................................
Advanced Courses and Seminars II
Analysis and Interpretation of Next-Generation Sequencing data
Coordinator(s): Marta Ferreira and Carla Oliveira
Faculty and invited speakers: Joaquin Jurado and Celina S. José
Short summary: Large-scale Sequencing projects have created a wealth of data for scientist that is worth to explore. However, the challenge is its analysis and even accessing these data to extract useful information connect to the system being studied.
This course focuses on employing Open-source bioinformatics software, tools and resources – including web-based programs, databases and common-line programs– to access and analyse the extraordinary volume of data available, to answer relevant biological questions. This course includes practical exercises for students.
Topics covered include the analysis of datasets obtained from high-throughput sequencing separated into 4 lectures.
Module I. How to explore and retrieve data sets from useful online bioinformatics databases (e.g. Ensembl, UCSC Genome Browser, C-BIOPORTAL, TCGA, GTEX, and others).
Module II. Genome Analysis. Analysis of datasets from DNAseq experiments to detect variants (Whole Exome Sequencing and Whole Genome Sequencing). From the mapping of sequencing data against the genome, to the calling of variants and annotation using multiple databases.
Module III. Transcriptome analysis (longRNA and smallRNA sequencing). Analysis of RNAseq datasets. Mapping against the genome, quantification of gene expression. Differential expression analysis and production of plots (heatmaps, boxplots) to represent results.
Module IV. Functional Enrichment and Network analysis.
Open-source bioinformatics software and tools.
Outline of the course: The course will span 4 days with a total of 20 hours with practical sessions and 4 modules (Aprox. 5 hours).
.......................................................................................................
COURSE: Mass Spectrometry-based Proteomics / Proteómica baseada em espectrometria de massa
Coordinator(s): Hugo Osório and Sara B. Pereira
Faculty and invited speakers: Hugo Osório, Sara B. Pereira, Celso Reis
Short summary: Recent developments in the field of proteomics have revolutionized the way that proteins and their function are studied. This course provides an overview of the most relevant Mass Spectrometry-based Proteomics (MS-proteomics) techniques, from experimental design, to sample preparation and data analysis. After the course, the students will be able to identify the critical steps and successfully design MS-based proteomics experiments.
Outline of the course: The course includes lectures on MS-based proteomic techniques, tutorials on experimental design, sample preparation and data analysis. Practical sessions will focus in the identification and quantification of proteins and characterization of protein post-translational modifications based on real data (18h).
.......................................................................................................
COURSE: Scientific Writing / Escrita Científica
Coordinator(s): Anna Olsson
Faculty and invited speakers: Anna Olsson, Júlio Borlido Santos, Helder Maiato, Alan Weed
Short summary: This course addresses the different steps and aspects in the process from research results to published paper.
Outline of the course: Lectures, exercises, group discussions.
.......................................................................................................
COURSE: Exploring the Cell - Light Microscopy Beyond Basics
Coordinator(s): Paula Sampaio
Faculty and invited speakers: Paula Sampaio, Maria Azevedo, Mafalda Sousa
Short summary: Light microscopy is a major technology in cell biology research. The course aims to introduce the students into state-of-the-art of light microscopy technology. Beside the basic concepts, it will be focus in the advanced light microscopy techniques and applications, such as optical sectioning methods, live cell imaging, molecular analysis, super-resolution microscopy, correlative light-electron microscopy and digital image analysis.
Course taught within the framework of an Open Training Course and application is required.
Outline of the course: lectures, tutorials, labs/practical classes (32h).
.......................................................................................................
COURSE: Histology and Electron Microscopy / Histologia e Microscopia eletrónica

Coordinator(s): Rui Fernandes
Faculty and invited speakers: Fernanda Fidalgo, Cláudia Pereira, Rui Fernandes, Cláudia Machado, Rossana Correia, Nuno Mendes
Short summary: The module is centered on Transmission Electronic Microscopy (TEM) namely conventional ultrastructure; immunoelectromicroscopy; elemental analysis – EDX; STEM. Training in tissue preparation, and Optical Microscopy namely in tissue preparation and equipment usage for cryomicroscopy. Fixation, embedding, sectioning and staining for LM applications. Principles of Immunohistochemistry.
Outline of the course: Lectures, tutorials and labs/practical classes (20h).
.......................................................................................................
COURSE: Bioinformatics / Bioinformática
Coordinator(s): Jorge Vieira and Cristina Vieira
Faculty and invited speakers: Jorge Vieira, Cristina Vieira, Hugo Lópes Fernandez
Short summary: Genome and transcriptome sequence data is accumulating at an explosive pace. For instance, more than one thousand animal genomes are now available, and there are hundreds or even thousands of gene sequences available for the most studied viral species. Such information can be used to address many biological problems. Therefore the students will learn how to get the data into the appropriate format for running the needed phylogenetic, positive selection, polymorphism, recombination and other analyses.
They will use software applications developed by us such SEDA (SEquence DAtaset builder; http://www.sing-group.org/seda), ADOPS (https://sing.ei.uvigo.es/ADOPS), BDBM (www.sing-group.org/BDBM/ ), as well as standard bioinformatics tolls.
Outline of the course: Lectures, practical classes (20h)
.......................................................................................................
COURSE: qPCR and gene expression analysis / PCR quantitativo em tempo real
Coordinator(s): Marta Vaz Mendes and Catarina Pacheco
Faculty and invited speakers: Marta Vaz Mendes, Catarina Pacheco
Short summary: Quantitative real-time PCR (qPCR) remains as the gold standard for targeted gene expression analysis. This course provides an overview of the qPCR methodology covering different aspects of a qPCR experiment from assay design to data analysis following the MIQE guidelines. The course provides the skills to identify the critical steps of a qPCR experiment that impact the quality and robustness of the experiment.
Outline of the course: The course includes lectures on the qPCR theoretical background, tutorial on the selection and validation of reference genes and a practical session on gene expression analysis based on real data (12h).
.......................................................................................................
COURSE: Protein expression, Purification and Characterization / Expressão, Purificação e Caracterização de Proteínas
Coordinator(s): Frederico Silva
Faculty and invited speakers: Frederico Silva, Fátima Fonseca, Margarida Bastos, Tony Rodrigues
Short summary: Proteins do most of the work in the cells and intercellular matrices of tissues, being required for all life processes including the catalysis of reactions, the regulation of pathways and the structural properties and movement of molecules, organelles and cells. This course will cover comprehensively the productions and purification of recombinant proteins and a wide range of protein characterization approaches. Technologies used in this context include: Chromatography, Ultracentrifugation, Spectrofluorimetry, Circular Dichroism, Surface Plasmon Ressonance, Isothermal Titration Calorimetry.
Outline of the course: The course will span a week (5 days) with practical sessions (Aprox. 16 hours) and 7 lectures (7 hours)
.......................................................................................................
COURSE: Structural Biology / Biologia Estrutural
Coordinator(s): Pedro J. B. Pereira and Sandra de Macedo Ribeiro
Faculty and invited speakers: Pedro J. B. Pereira, Sandra de Macedo Ribeiro
Short summary: This course will introduce the students to the field of Structural Biology and guide them through thinking about biological problems in 3D. It will include training on methods used to visualize and interpret macromolecular structures and an overview of the experimental flowchart used to determine 3D structures by X-ray crystallography, NMR and Cryo-Electron Microscopy. An overview on protein crystallization, data collection and processing, structure refinement and model building will be provided using the equipment and facilities available at i3S.
Outline of the course: The course will consist of tutorials focusing on analysis of structural biology papers, crystallography data collection, analysis and 3D structure interpretation (16h).
.......................................................................................................
COURSE: Multicolor Flow Cytometry / Citometria de Fluxo
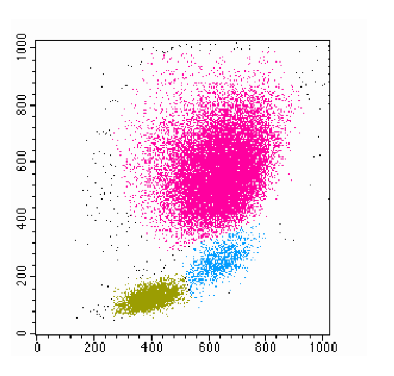
Coordinator(s): Catarina Meireles and Emilia Cardoso
Faculty and invited speakers: Catarina Meireles, Emília Cardoso
Short summary: Flow Cytometry is a laser-based technology that permits analyzes of expression of cell surface and intracellular molecules, characterizing and defining different cell types in a heterogeneous cell population. It is mainly used to measure fluorescence intensity produced by fluorescent-labeled antibodies detecting proteins, or ligands that bind to specific cell-associated molecules. This is a powerful tool which allows a simultaneous multiparametric analysis of the physical and chemical characteristics of up to thousand particles per second. Thus, a number of applications, including the multicolor analysis of cell phenotype, gene expression, membrane potential, Ca2+ and Mg2+ influx and cell cycle can be performed. Additionally, you can purify subpopulations of interest in high-speed and high-purity (up to 99.0%) by multi-laser cell-sorting.
Outline of the course: 15h
| DAY 1 | Morning | Flow cytometry basis |
| Afternoon | Design experiment | |
| DAY 2 | Morning | Hands on cytomers (Practical) |
| Afternoon | Data Analysis (FlowJo Software) |
.......................................................................................................








